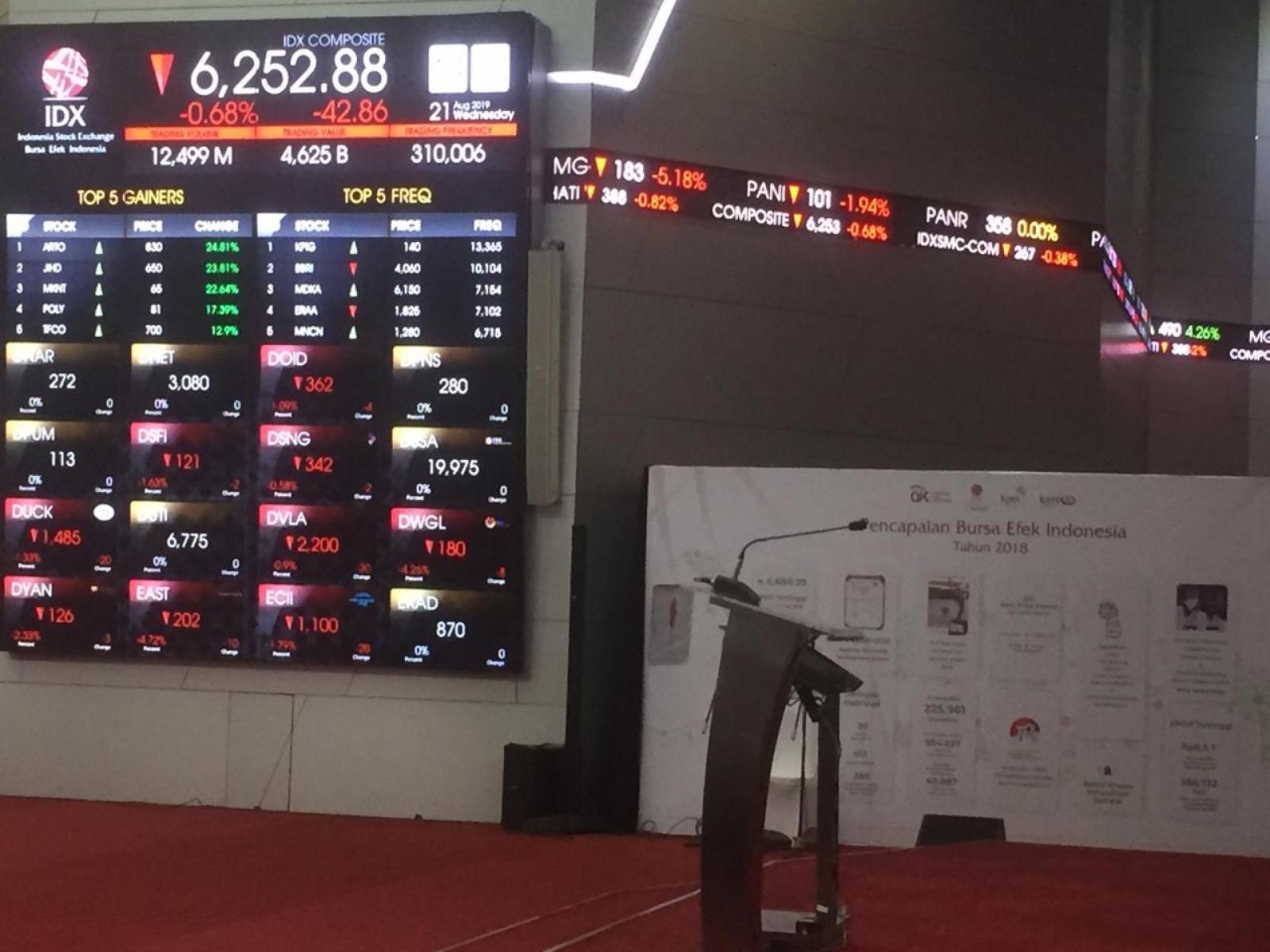
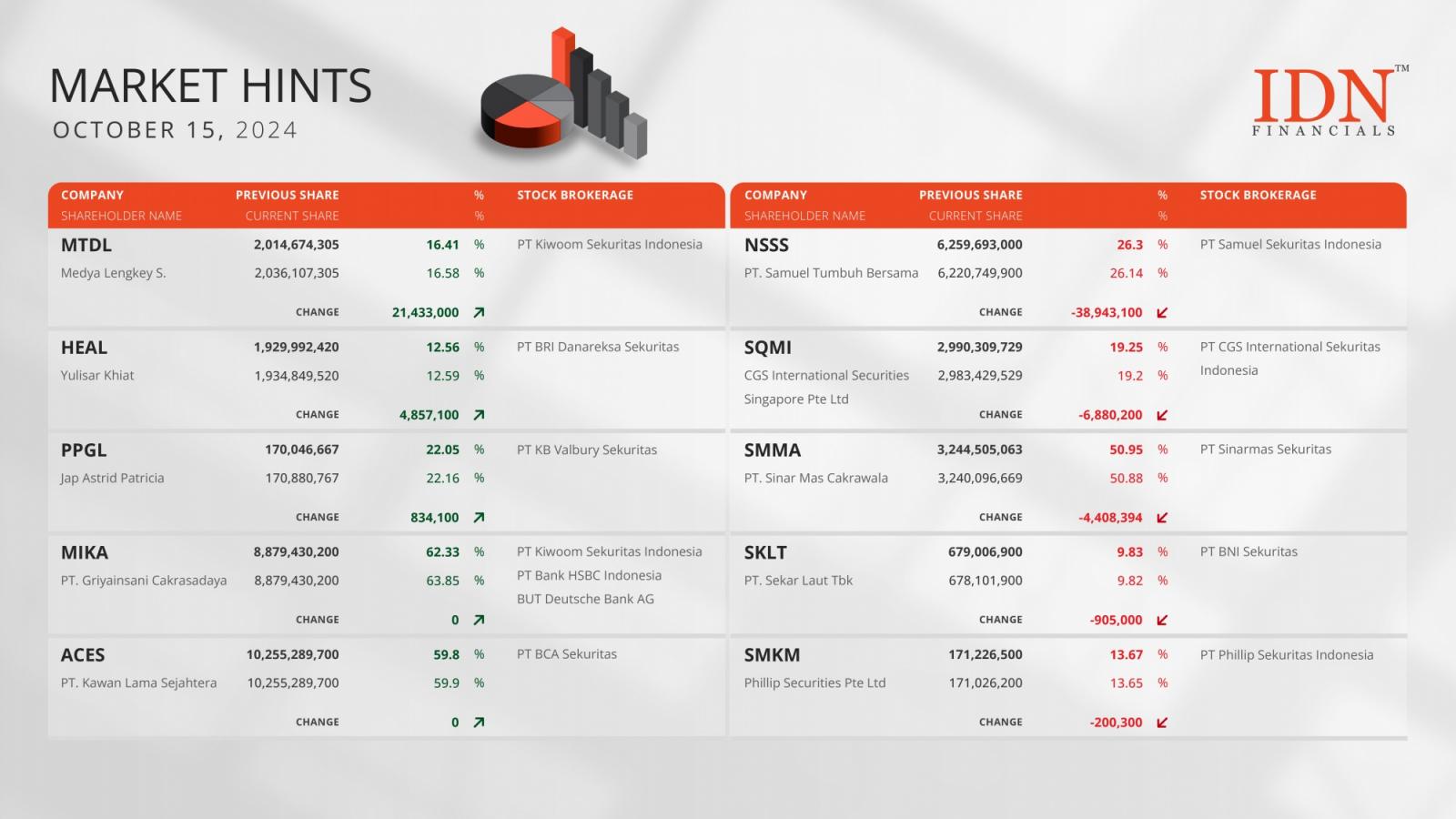
Binance to Delist Multiple TrueUSD Trading Pairs – What's Going On?
Binance, the world’s largest cryptocurrency exchange, has announced its plan to delist multiple TrueUSD spot trading pairs as well as a couple of BNB pairs, its native currency. The notice published on Wednesday, March 13, revealed that Binance will remove TrueUSD and BNB pairs that will include PENDLE/TUSD, COMP/TUSD, and EDU/TUSD. Additionally, the BNB duo...